
The skin is our largest organ.
The skin is unique in many ways, but no other organ is so visible and demands so much attention and care in both diseased and healthy states.



About us
Dermaliq Therapeutics, Inc., is a dermatology and aesthetics company with a rich pipeline focused on follicular diseases and conditions. We are developing differentiated medical aesthetics, self-pay prescription, and OTC products.
Dermaliq’s mission is to develop a new generation of high-quality and user-friendly products with maximum efficacy and safety. Our unique “hyliQ®” technology overcomes the “delivery barrier” and for the first time enables the adsorption of multiple attractive active ingredients and drugs into the follicle and skin, enabling the development of “first in class” and “best in class” treatment options.
Medical dermatology, aesthetics and follicular diseases
Skin and hair disorders are a leading cause of nonfatal disease burden worldwide and have a significant impact on a person’s well-being, mental health, ability to function, and social participation.
Topical, localized delivery of active ingredients to skin and scalp is the preferred route of treatment in most patients, reducing the risk of systemic side effects. Dermaliq’s transformative hyliQ® technology ushers in a new generation of topical products which offer not only superior dermal drug delivery for conditions like psoriasis and eczema but targeted delivery deep into hair follicles and sebaceous glands.
Specifically, hyliQ® technology consists of novel lipophilic excipients with lowest viscosity and surface tension which enables rapid penetration into follicles and the formation of active ingredient depots in the associated glands. This opens new avenues for topical treatment of many challenging dermatological conditions, including:
- Alopecia: Hair loss that can occur in various patterns, including alopecia areata (patches of baldness) or androgenetic alopecia (male or female pattern baldness).
- Folliculitis: Inflammation of hair follicles, often caused by bacterial or fungal infections.
- Hirsutism: Excessive hair growth in women in areas where men typically grow hair.
- Acne vulgaris: A condition where pimple-like bumps or keloid scars (comedones, papules, pustules, nodules) develop in the face or on the back of the neck.
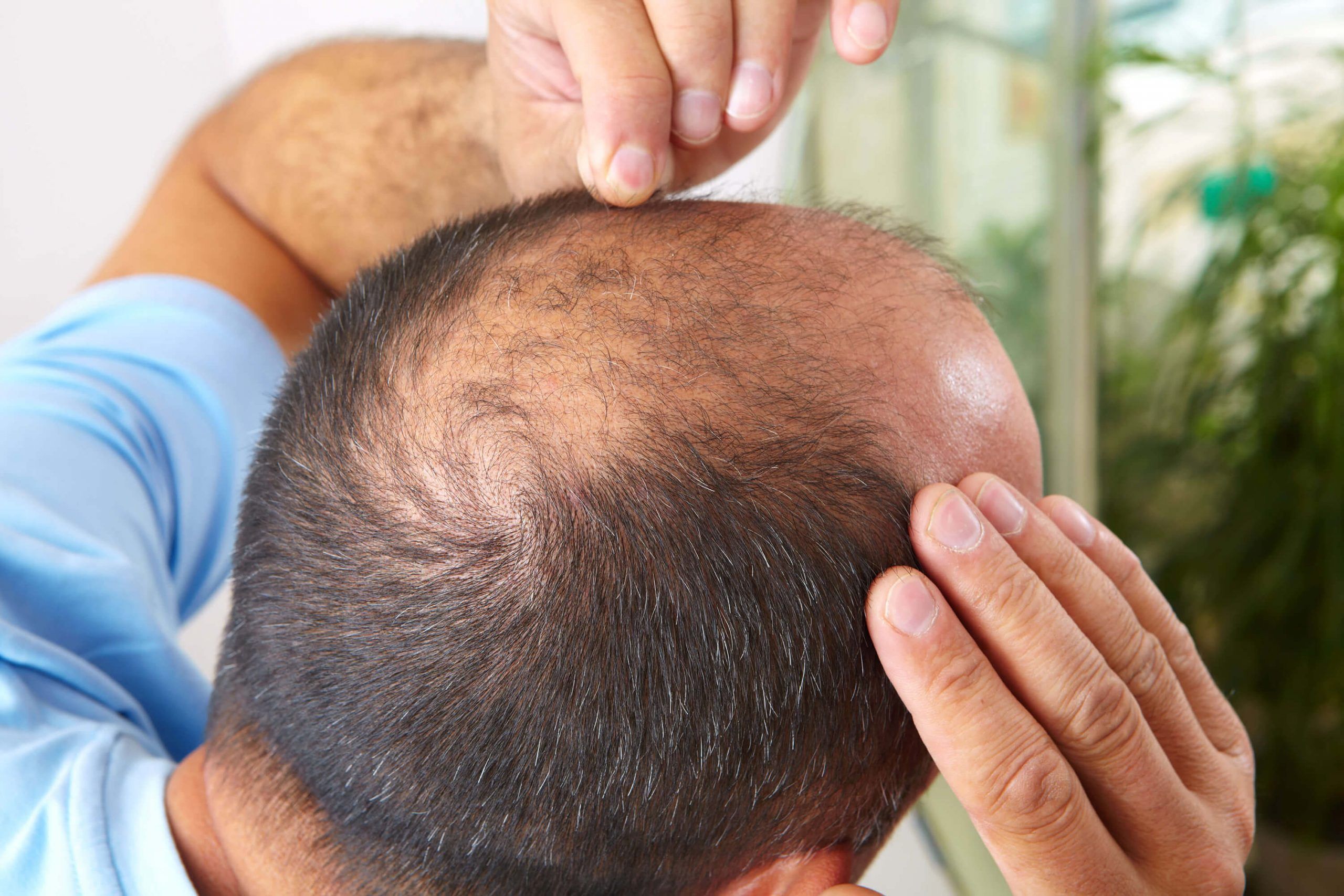
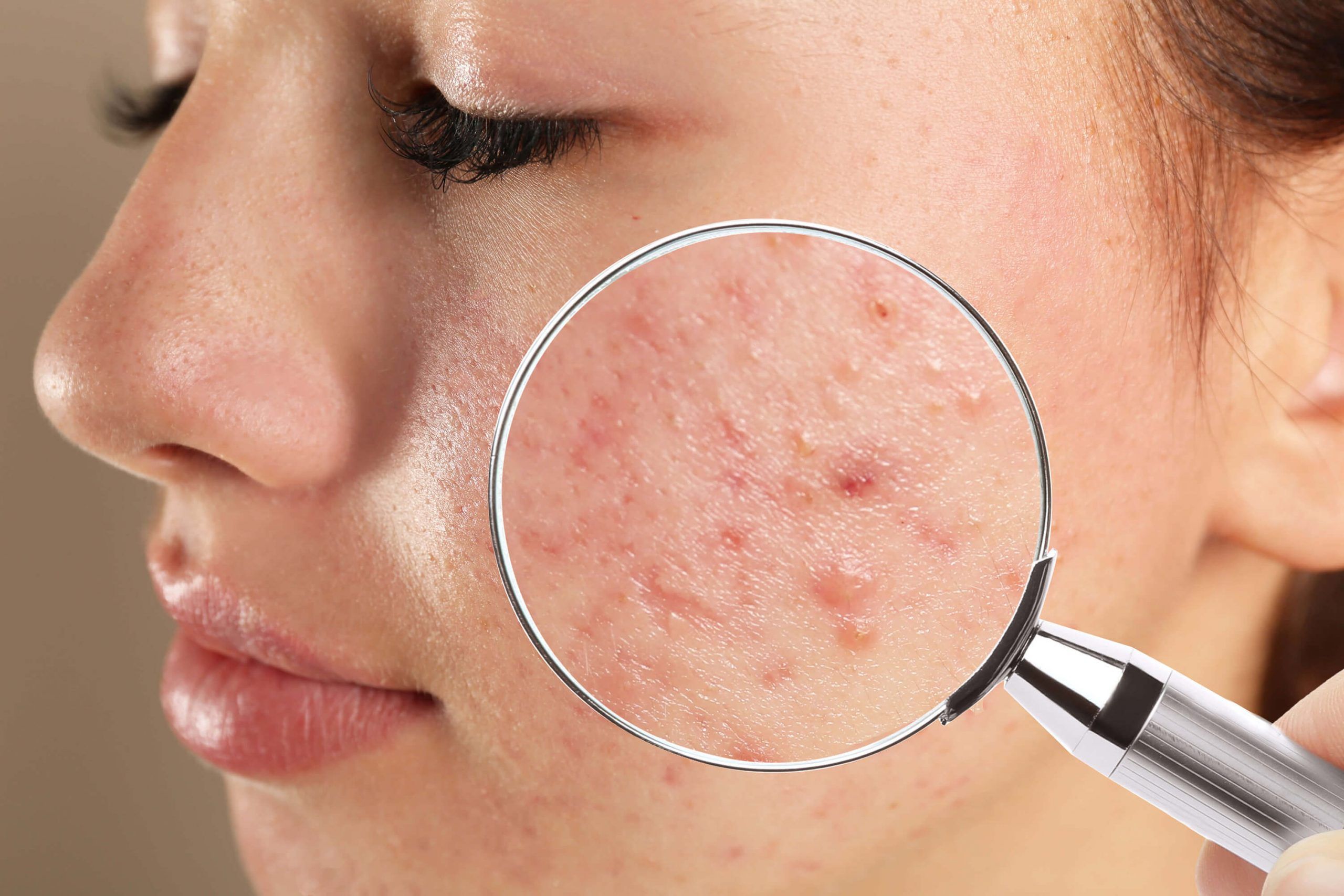
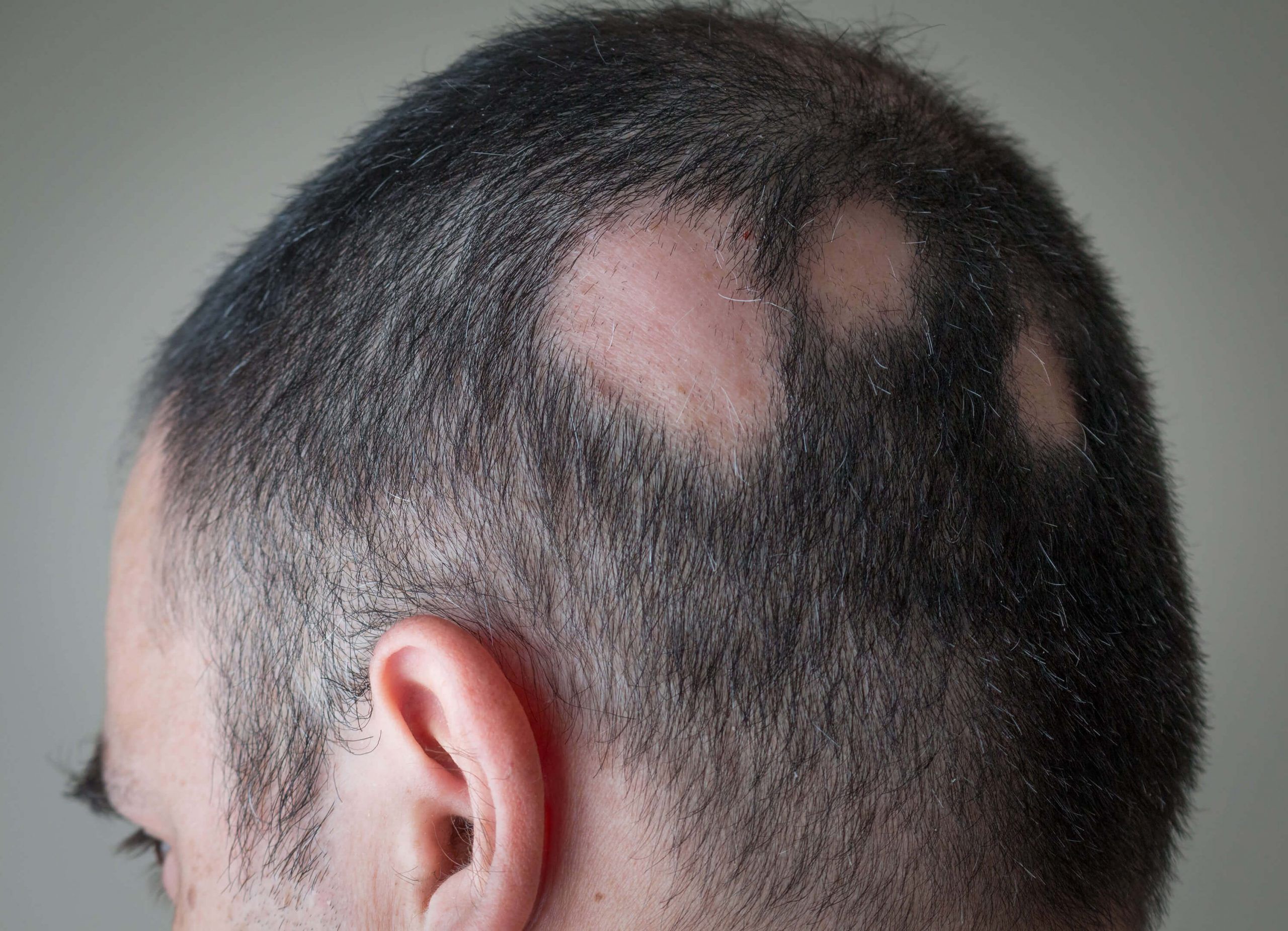
Technology
The evolutionary hyliQ®– technology
hyliQ®, the unique and proprietary technology owned by Dermaliq, is designed to provide cutaneous drug delivery with unmatched bioavailability for a wide range of pharmaceutical actives.
The technology enables the development of superior liquid therapeutic drugs and medical skin care products which are characterized by exceptional cosmetic properties.
hyliQ® products do not require skin irritating excipients such as preservatives or penetration enhancers.


How it works
Proprietary hyliQ® carrier molecules transport solubilized active ingredients through the stratum corneum, preferably into the epidermis, into glands, or into hair follicles. By selection of specific carrier molecules, we can customize the properties of our drug products, enhancing targeted penetration into the skin tissues, increasing efficacy and reducing unwanted side effects.
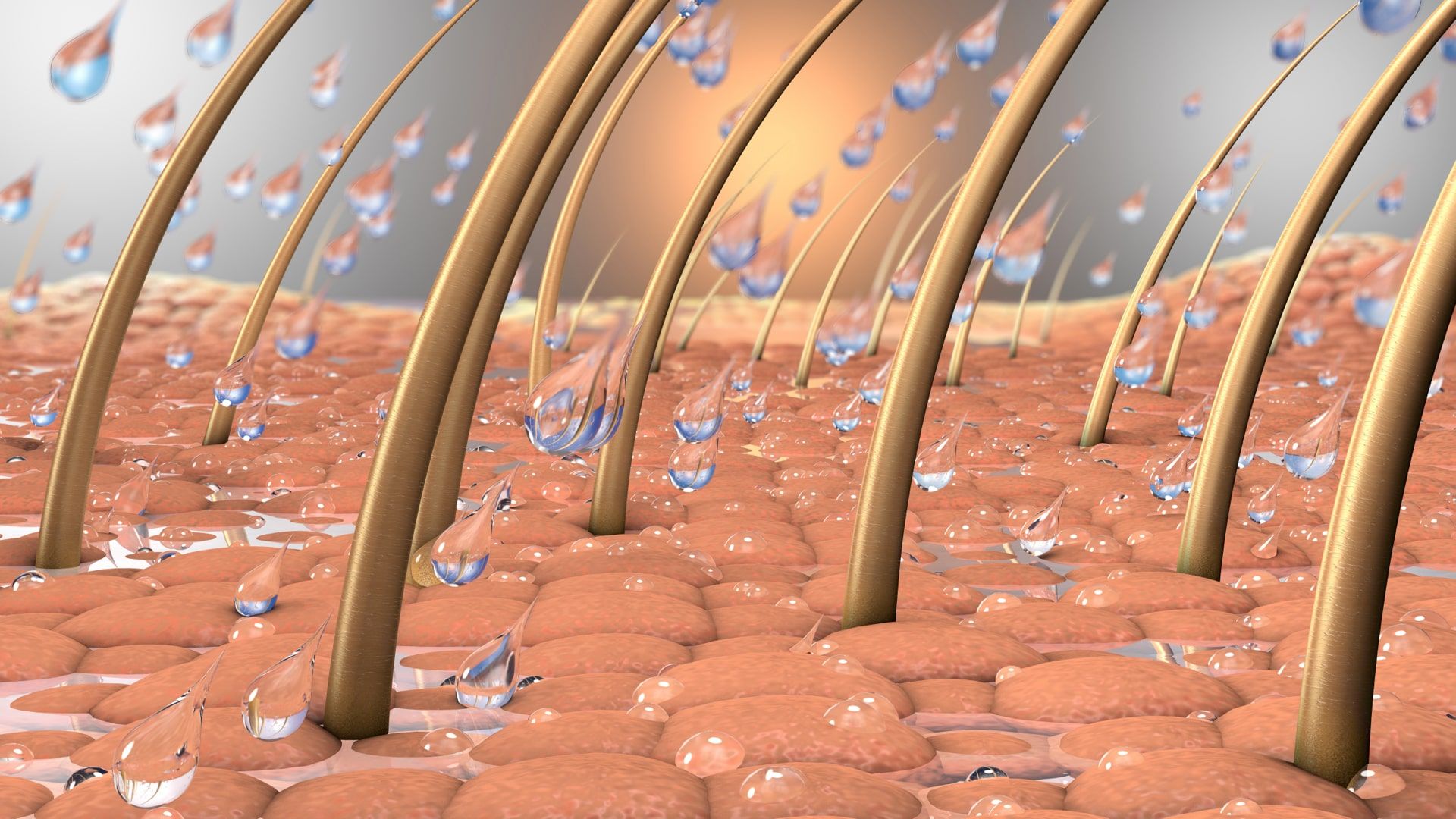
Our Pipeline
Our goal is to establish a comprehensive pipeline of next-generation topical drugs and medical skin care products based on our proprietary hyliQ® technology.
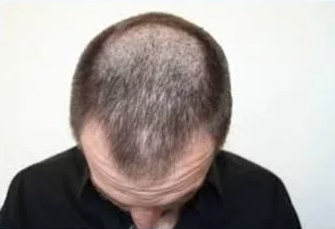
DLQ01
-
Androgenetic Alopecia
DLQ01 has the potential to become the leading next generation hair growth product. It is a liquid topical formulation of a prostaglandin (PG) F2alpha analog in hyliQ® designed to stimulate scalp hair growth in men and women suffering from androgenic alopecia, also known as male and female pattern baldness.
DLQ01 Phase 2 data has impressively demonstrated its superior efficacy. The number of hairs in the target area increased effectively with high statistical significance compared to the vehicle. More than 80% of subjects responded with a positive change in hair growth. We are continuing clinical development based on a clear regulatory pathway to approval.
| Preclinical | Phase 1 | Phase 2a | Phase 2b | Phase 3 | |
|---|---|---|---|---|---|
|
|
|||||
|
|||||
DLQ01
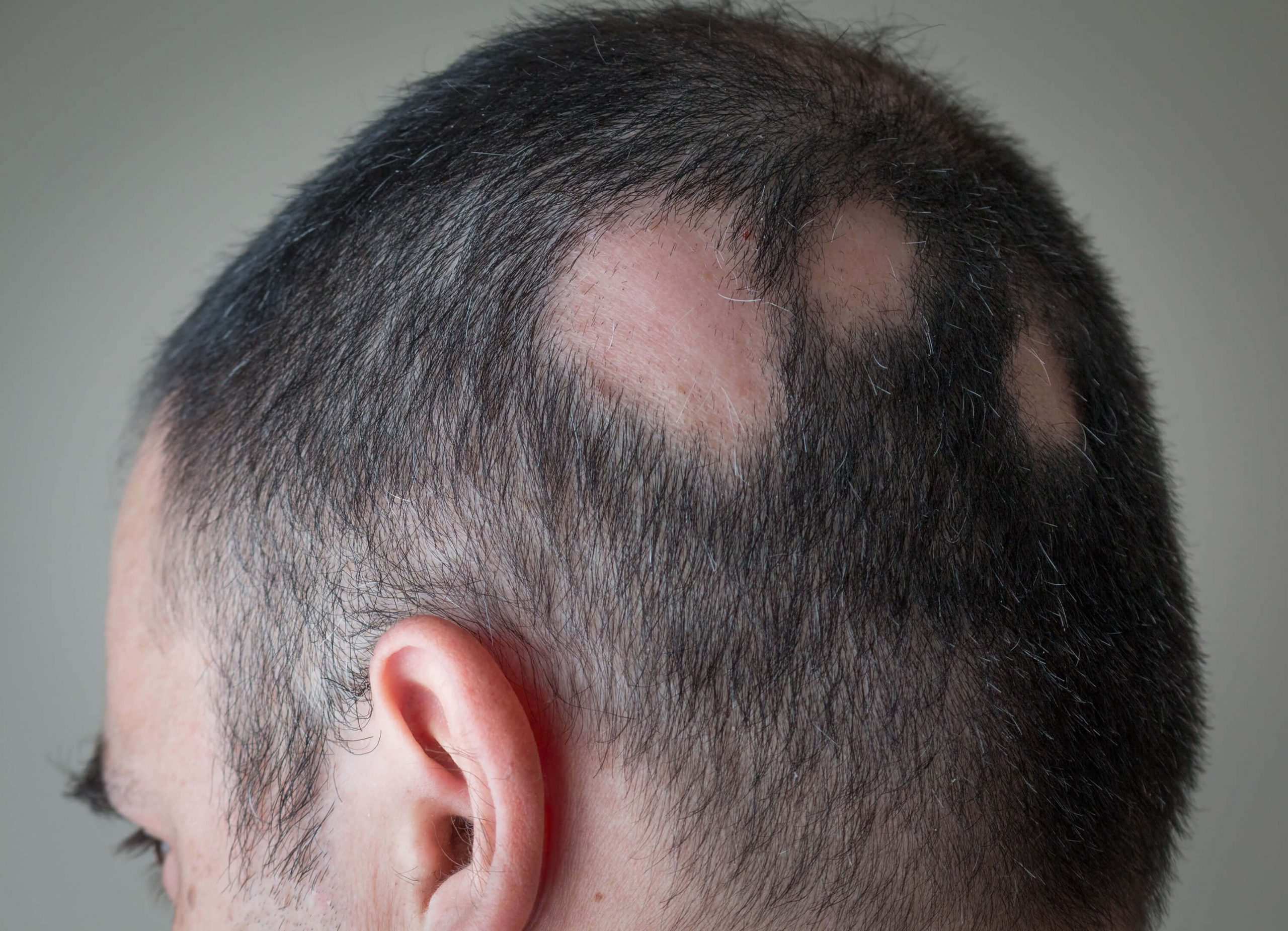
DLQ02
-
Alopecia Areata
Alopecia Areata (AA) is a chronic immune-mediated condition affecting up to 2% of the global population.
It is characterized by autoimmune, chronic, and relapsing hair loss ranging from scattered patches to complete loss of hair, with significant psychological impact. At present, there are only two approved systemic drugs, which have a limited response rate. There is a high unmet medical need for safe topical applications, especially for mild and moderate forms of AA that only affect small areas of the scalp.
DLQ02 is a liquid topical, calcineurin inhibitor with anti-inflammatory and immunomodulating properties, developed for the treatment of immune-mediated conditions. DLQ02 offers a dual mode of action, combining immunomodulation with hirsutism, which promotes additional hair growth.
We plan to initiate a phase 2a study in Alopecia Areata by Q4/2024
| Preclinical | Phase 1 | Phase 2a | Phase 2b | Phase 3 | |
|---|---|---|---|---|---|
|
|
|||||
|
|||||
DLQ02

DLQ03
-
Acceleration of wound healing and prevention of secondary skin infections
DLQ03 is a differentiated topical drug candidate, targeting accelerated wound healing and the prevention of secondary infections and to support the injured skin barrier. Burn wounds, but also artificial wounds after aesthetic procedures such as lasers, chemical peels, injections and surgery etc. are invasive and carry the risk of secondary infections and scarring.
DLQ03 Phase 2 data in human impressively demonstrated its superior efficacy in accelerating wound healing which was achieved 20-25% faster compared to vehicle.
| Preclinical | Phase 1 | Phase 2a | Phase 2b | Phase 3 | |
|---|---|---|---|---|---|
|
|
|||||
|
|||||
DLQ03
Management and Board of Directors

Christian Roesky, PhD
CEO & President Dermaliq Inc
Director & Chairman
Director
Dr. Roesky is CEO & Managing Director of Novaliq since November 2016. Previously, Dr. Roesky was General Manager for the D-A-CH region of Bausch + Lomb GmbH in Berlin; Commercial Director Central Europe of Abbott’s Diagnostics Division, General Manager and speaker of the German Country Management Board of Abbott GmbH & Co. KG in Wiesbaden from 2013 – 2015; and, General Manager of Alcon Germany & Austria (Novartis) from 2009-2013. He studied chemistry in Bonn and Freiberg, both in Germany, and was awarded his Ph.D. with honors from the Technical University of Freiberg in Germany.

Oliver Schlüter, PhD
CFO & Co-Founder Dermaliq Therapeutics Inc.
Director (Chair)
Dr. Schlüter is the CFO and a co-founder of Dermaliq. He serves as Dermaliq’s Chairman of the Board of Directors. In parallel, Dr. Schlüter continues serving as Novaliq’s CFO, a position he has held since 2015. Prior to this Dr. Schlüter was CFO of CureVac GmbH from 2010 to 2015 where he was instrumental in the transformation of a 70 employee R&D-driven company to a 170-employee customer-centric and value-focused corporation that entered into several strategic partnerships with in-house cGMP-manufacturing. During his time at CureVac the company’s value increased more than tenfold and reached unicorn status with a value of more than $1bn. During the past 15 years in Biotech/Pharma he secured more than $400m cash for the organizations he served. Dr. Schlüter has 25+ years of professional experience within the fields of Finance and Life Sciences. He holds a PhD in computational finance and also studied business. administration.

Karen Liu, PhD
Founding partner 3E Bioventures Capital
Director
Dr. Karen Liu has been active in healthcare venture investments since 2005. She is a founding partner of 3E Bioventures Capital, a life science focused VC firm which actively invests in innovative therapeutics as well as cross-disciplinary health tech innovations. 3E has invested in more than 80 companies since its inception in 2015. Prior to a career in investment, Dr. Liu had a successful Internet start-up experience and had also worked as a consultant at Mckinsey & Company.
Dr. Liu received a Ph.D. in Immunology from Harvard University, Master of Medical Sciences degree from Harvard Medical School, and Bachelor of Sciences degree from Cornell University. She also received an EMBA degree from CKGSB – Chungkong Graduate School of Business in China.

Robert J. Moccia
Independent Director
Robert (Bob) J. Moccia (Independent Board Director) is an entrepreneurial leader with 22+ years of executive management experience as President, COO, and CEO of small to mid-sized specialty pharmaceutical and Dermatology companies. He has held the following positions, among others:
President & CEO of Strata Skin Sciences, President; CEO & Co-Founder of Encore Dermatology,
President & CEO of Precision Dermatology, Inc., President of Onset Dermatology, LLC and SVP Corporate Development at Medicis Pharmaceuticals Inc..
Mr. Moccia is a recognized expert in development, commercialization and marketing strategies.

Betsy Hughes-Formella, PhD
CSO & Co-Founder Dermaliq Therapeutics Inc.
Dr. Hughes-Formella is CSO and co-founder of Dermaliq. She has almost three decades of experience in the development of of topical products and transdermals for a wide range of indications including psoriasis, atopic dermatitis, acne, rosacea, skin infections, pain, wound healing, and aesthetic products. Prior to joining Dermaliq, Dr. Hughes-Formella provided independent consulting services to the global pharmaceutical and CRO industry for 5+ years after holding a management position in a niche CRO specialized in the conduct of dermatological trials for 22+ years. She earned her MS and PhD at the Department of Physiology and Pharmacology, College of Veterinary Medicine, University of Georgia, Athens.

Frank Löscher, PhD
Co-Founder Dermaliq Therapeutics Inc.
Dr. Löscher is the CEO, President and co-founder of Dermaliq. Prior to joining Dermaliq, he was Chief Technology Officer of Novaliq GmbH, an ophthalmic drug development company. There he was instrumental in the development of several drug candidates and products such as NOV03, CyclASol, EvoTears/NovaTears. He is inventor and co-inventor of numerous drug candidates and developed the pipeline and business case for Dermaliq. Dr. Löscher graduated as a chemist from the University of Heidelberg and received his Dr. rer. nat. degree from the University of Regensburg.
Scientific and Medical Advisory Board

Michal E. Kuligowski, MD, PhD, MBA, iFAAD
VP, Dermatology, Clinical Research Group at Thermo Fischer Scientific, Inc.
Advisory Board
Dr. Kuligowski is an experienced pharmaceutical-industry dermatologist with 12 years of clinical practice in dermatology – primarily in an academic setting – a doctoral degree in this specialty, and a business degree to supplement his scientific/ clinical education.
Dr Kuligowski has 25+ years of experience in the pharmaceutical companies (10 years in medical affairs and 16+ in clinical development) including a variety of regional and global roles in larger and smaller companies across Europe, North America, Middle East, and APAC. He has worked across all phases of clinical drug development of small molecules, biologic agents and topical drugs with additional exposure to dermatological aesthetic products.
Dr. Kuligowski earned his doctorate and medical degree from the Medical University of Warsaw in Warsaw, Poland, and a MBA from Webster University in St. Louis, MI.

Ralf Paus, MD, DSc, FRSB
Research Professor of Dermatology at the University of Miami Miller School of Medicine
Advisory Board
Ralf Paus, MD, DSc, FRSB, is a German academic dermatologist with > 35 years of experience in clinical dermatology and skin and hair research (>700 PubMed-listed publications, H-index: 117). After post-doctoral studies at Yale University, New Haven, CT, during which he became fascinated by the biology and pathology of the hair follicle, dermatology residency training in Berlin and a junior faculty position at the Charite University Hospital, Berlin, his last clinical appointment was a Professor and Vice Chair, Dept. of Dermatology, University Hospital Hamburg-Eppendorf (1999-2004). Since then, Ralf has focused on translational hair research and skin neuroendocrinology, first as Head of Experimental Dermatology, University of Luebeck, then as Director of Research, Centre for Dermatology Research, University of Manchester (where he is now the Emeritus Professor of Cutaneous Medicine), and since 2018 as Research Professor of Dermatology at the University of Miami Miller School of Medicine, Miami, FL, USA. Ralf has served as Editor of Experimental Dermatology (2007-2021) and is a serial entrepreneur, most recently as Founder & CEO of CUTANEON – Skin & Hair Innovations, Hamburg & Berlin, Germany.

R. Todd Plott, M.D.
Chief Medical Officer Epiphany Dermatology
Advisory Board
R. Todd Plott, M.D., is a board-certified dermatologist in the Dallas/Fort Worth area and Chief Medical Officer of Epiphany Dermatology with 30 years of experience in clinical dermatology. Before entering private practice, Dr Plott spent 16 years in the pharmaceutical industry developing several dermatology drugs widely prescribed by dermatologists, his most notable invention is Solodyn™. Dr. Plott obtained his medical degree from the University of Texas Medical Branch in Galveston.

Xavier Yon
Former CEO Galderma
Advisory Board
Xavier Yon, is former CEO of Galderma and a seasoned healthcare executive with an extensive background in dermatology. Previous roles include executive positions with Pfizer, Solvay, Alcon and most notably as Chief Executive Officer of Galderma for 17 years. During his time at Galderma Mr. Yon built the company from its initial spin out from Alcon to be a world leader in ethical dermatology. Mr. Yon holds a number of active board and advisor roles. He holds a degree in Physics, Chemistry and Biology from Sorbonne University, Paris.
Contact us
Head Office
Dermaliq Therapeutics, Inc.,
1201 North Market Street, Suite 111,
Wilmington, DE 19801, USA
German Subsidiary
Dermaliq Therapeutics GmbH
Im Neuenheimer Feld 515
69120 Heidelberg, Germany
